| Abstract | Introduction | Materials and Methods | Results |
|---|---|---|---|
| Discussion | Conclusions | Acknowledgments | References |
| Fig. 1 | Fig. 2 | Fig. 3 | Fig. 4 | Fig. 5 | Fig. 6 | Fig. 7 | Fig. 8 | Fig. 9 | Fig. 10 |
|---|
| Table I | Table II | Table III | Table IV |
|---|
Powerful new mathematical approaches allow examination of subtle features of Drosophila melanogaster wing shape. Planar geometry of wing landmarks of three established wild type strains of D. melanogaster shows that each of 13 landmarks studied, including the major intersections of wing veins I, II, III, IV and V with crossveins and the wing margin, are affected similarly by sex in each strain. This sex dimorphism is conserved to a variable extent in Drosophila species of a separate subgenus. Neither confidence ellipses nor centroids for those dimorphic landmarks in the two sexes are able to be superimposed by translation, rotation or isometric scaling. Thin plate splining of the three wild type strains resolves three principal components of variability of wing shape that have parallel, similarly directed sexual components. The major difference is expressed as an expansion of the distal wing region in males compared to females. Thus an affine transformation does not eliminate the sex dimorphism when applied to the raw data or the average differences. There is no detectable interaction between sex and strain within the D. melanogaster species. This may allow future strategies for identifying the genes involved in these subtle shape differences. Using discriminant scores, based on all 13 landmark confidence ellipses, correct sexual membership is assigned for approximately 90% of males and females. Temperature shift experiments using the transformer-2 temperature sensitive allele-2, tra2ts2 demonstrate that the sexual differences in wing shape are established during the imaginal disc growth phase of larval development. Temperature shifts from the non permissive temperature (29°C) to the permissive temperature (18°C) result in 100% female-to-male wing transformation if made after 1-day prior to larval wandering at 29°C. While the shape of wings is clearly regulated via the tra2 pathway, the size of the wing blade remains related to the sex-chromosome to autosome (X:A) ratio. Regional differential expression of growth factors during the imaginal disc growth phase could account for sex dimorphism.
Sexual dimorphism in Drosophila melanogaster is regulated via gene dosage and specific gene action (Slee and Bownes, 1990; Steinmann-Zwicky et al., 1990). The ratio of sex chromosomes to autosomes (X:A) determines the differential activity of sex-lethal (Sxl) by blastoderm stage of the embryo via several identified genes (maternally acting daughterless (da) and Daughter killer (Dk), zygoticly acting sisterless a and b (sis-a and sis-b), virilizer (vir), male-specific lethals 1,2,3 (msl 1,2,3), maleless (mle) and hermaphrodite (her)). While the X:A ratio sets the primary signal for sex by its effect on Sxl, the specific regulation of tissue differentiation is carried out later at specific times (Epper and Bryant, 1983) by a resultant cascade of sex specific activations of a series of identified subsidiary genes: doublesex (Dsx), intersex (ix), transformer (tra) and transformer-2 (tra2). The activities of these five genes (Sxl, Dsx, Ix, tra and tra2) at the cellular level are thought to regulate the coordinate morphological, biochemical and behavioral differences that constitute observed maleness and femaleness. Temperature shift experiments, using the temperature sensitive allele tra2ts2, determined that the temporally specific expression of genes associated with the development of sexually dimorphic genital morphologies occurs in the pupal stage (Epper and Bryant, 1983) While various aspects of Drosophila cuticular color and structure have been shown to be sexually dimorphic (MacDougal et al., 1995), the most significant difference in wing structure dimorphism that has been demonstrated to date has been an X-linked component of wing variability shown to be related to dosage compensation (Cowley et al., 1986).
To what extent all subtle sexually dimorphic traits in adults are controlled by the latter group of five genes is not known. In this study we show that we can detect sex dimorphism in wing shape and size using planar geometry of wing venation landmarks. The shape and size parameters of wings are separable: Shape is shown to be regulated by tra2 acting in the larval imaginal disc growth period, while size remains correlated with X:A ratio. We further demonstrate that sex and strain effects have no significant interaction in the wild type strains examined.
Animals
Cultures of Drosophila (Sophonophora) melanogaster (Porch
1984, 86, 88 and Univ. Tennessee Ag. Peach 1966), Drosophila (Drosophila)
virilis (Markert Apple Storage 1967) and Drosophila (Drosophila)
immigrans (Pelham, 1988) were obtained from the culture collections
of the late Phil Ives of Amherst College (Ives, 1970).
D. (S.) melanogaster (Canton S) was obtained from Rod Murphy
of the University of Massachusetts. A culture of D. melanogaster
containing transformer2 (tra2ts2 ) balanced by
the In(2LR)CyO, dplvICy pr cn chromosome (CyO) was obtained from Mary Bownes
of Edinburgh, Scotland. Homozygotes of tra2ts2
segregate from crosses of Cy tra2+/Cy+tra2ts2 and were
recognized by their non-Curly phenotype. In tra2 temperature
shift experiments chromosomal males were identified by being Bar eyed due
to a small duplication on the Y carrying the dominant marker, Bar of Stone
(Belote and Baker, 1983).
Wing preparation and measurement
Wings were obtained from sexed individuals, dehydrated in isopropanol
and limonene and mounted in Permount on microscope slides. Wings
were imaged with an 8-bit 640x480 square pixel camera (CCD-72A) mounted
on a Zeiss IM35 with a 2.5x objective and grabbed with a Matrox PIP-640
graphics card installed in a 386 MsDos computer. The CCD array obtains
its information from the central half of the microscopic image field and
does not include any distortion of square stage micrometer reticule arrays
which we used to calibrate the images. The images were stored over
Ethernet onto a 1 Gbyte disc drive mounted on a SPARCstation® 10 (Sun
Microsystems) and archived onto a tape unit on that computer. Custom
software running on 386 or 486 MsDos computers was used by trained operators
to locate landmarks of the wing images. Rules were established defining
the center of each landmark at the resolution of a horizontal wing filling
a 640x480 screen, Fig 1. The simplistic
rule, followed to the best of each operators ability, was to imagine
the wing veins to be roadways and center the landmark on an imagined traffic
island, optimally placed at each landmark to allow traffic flow as well
as safety to the operator standing on the island.
Image and Data Acquisition Software
Custom software for image grabbing and landmark extraction using
a mouse was written in Microsoft C 5.0 around a Microsoft C Library for
the Matrox PIP-640 8-bit 1Kx1K video board.
Data Analysis Software
Planar geometry of wing landmarks was carried out using software,
Planar 1.0, developed by the first author in Turbo Pascal 5.0 based on
the published algorithms of Siegel (1981).
The Planar 1.0 software uses a least square alignment algorithm in which
a set of k 2-D landmarks (ui, vi) of shape B, i =
1,..,k are aligned to k 2-D landmarks (xi, yi) of
shape A using the equation,
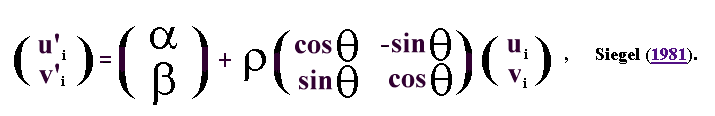
The aligned landmarks of B, B' = (u'i ,v'i)
, are obtained from the original landmarks (ui, vi)
by the action of the four parameters, ![]() ,
, ![]() ,
, ![]() and
and ![]() , which
are estimated by a least squares algorithm. The parameters
, which
are estimated by a least squares algorithm. The parameters ![]() and
and ![]() translate
the centroid of shape B to shape A. The parameter
translate
the centroid of shape B to shape A. The parameter ![]() isometrically scales shape B to the size of shape A. The parameter
isometrically scales shape B to the size of shape A. The parameter ![]() represents the angle used to rotate the landmarks of shape B onto those
of shape A within a least square residual of perfect alignment.
After alignment, landmark coordinates of n wings were averaged to create
a class average of landmarks and k 2x2 covariance matrices of the
aligned landmarks, covi. The group averages and covariance
matrices were used to calculate confidence ellipses for the landmarks of
grouped wings. Relative size of wings was captured in the independent
isometric scaling parameter,
represents the angle used to rotate the landmarks of shape B onto those
of shape A within a least square residual of perfect alignment.
After alignment, landmark coordinates of n wings were averaged to create
a class average of landmarks and k 2x2 covariance matrices of the
aligned landmarks, covi. The group averages and covariance
matrices were used to calculate confidence ellipses for the landmarks of
grouped wings. Relative size of wings was captured in the independent
isometric scaling parameter, ![]() .
The wing group confidence ellipses for landmarks comprised the bases of
a newly devised discriminant function analysis, which was used to predict
the group membership of individual wings.
.
The wing group confidence ellipses for landmarks comprised the bases of
a newly devised discriminant function analysis, which was used to predict
the group membership of individual wings.
The discriminant function for a class of wings (such as male Canton S) based on k landmarks assumes under the null hypothesis (H0: the wing belongs to this class) that each of the k wing land marks is independent of the other k-1 landmarks and is distributed bivariate normal in the plane of the wing with the computed mean and covariance matrix calculated from a basis sample of wings for that class. Then the log likelihood (LL) of being a member of this class is given by:
For the sake of computing a single numeric measure of difference between two shape classes approximations to Procrustes distances in Euclidean (shape) space were computed in tangent space using the tpsSmall program of Rohlf (1997C). Procrustes distances are inherently reciprocal and have been recommended for quantitative comparisons. The abstraction from Euclidean space to tangent space causes some question of interpretation. The program tpsSmall of Rohlf (1997C) was also used to demonstrate the high correlation between wing differences measured in shape space and tangent space. When the differences between shapes are small a high correlation computed by tpsSmall is a confirmation that Procrustes distance estimates, thin plate spline calculations and significance tests computed in tangent space are not distortions.
Factorial design experiment contrasting the two sexes, three strains and their potential interactions affecting wing shape were analyzed using tpsRegr. This requires representing the experimental contrasts of groups according to the General Linear Model: Y = Xß (Rao, 1965). To implement this in version 8 of tpsRegr one needs to construct a factorial design matrix, X, which selects parameters from the factor matrix ß to add up to the specimen landmark matrix, Y, and describes the membership of each wings landmarks in a particular strain and sex in the columns of the matrix using 0's, 1's or -1's. To facilitate this simple design matrix, an equal number of individuals needs to be chosen from each strain and sex within the six sex-strain combinations. The equal sample size in each treatment group, though not essential, makes the conceptualization and analysis simpler. An illustration, with a hypothetical minimally spanning sample of 6 wings, of the relationship of 6x2k observation matrix Y and 6x2k parameter matrix ß to 6x6 design matrix X is illustrated in the accompanying table:
|
|
|
|
ß = ( µ S1 S2 m/f S1xm/f S2xm/f )' | ||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
y10 = µ + S1 + m/f + S1xm/f |
|
|
|
|
|
|
|
|
|
|
y11 = µ + S1 - m/f - S1xm/f |
|
|
|
|
|
|
|
|
|
|
y20 = µ + S2 + m/f + S2xm/f |
|
|
|
|
|
|
|
|
|
|
y21 = µ + S2 - m/f - S2xm/f |
|
|
|
|
|
|
|
|
|
|
y30 = µ - S1 - S2 - m/f - S1xm/f - S2xm/f |
|
|
|
|
|
|
|
|
|
|
y31 = µ - S1 - S2 - m/f + S1xm/f + S2xm/f |
Calculations of thin plate splines which transform two or more different topographies with homologous landmarks into a common intermediate were calculated using the tpsRelw software of Rohlf (1997B). Superimposition and averaging of wing images was performed with the 32-bit version of tpsSuper Ver. 1.03 of Rohlf (1997D) on a 200 MHz Pentium Pro with 64 Mbyte RAM running Windows NT 4.0.
To eliminate the effects of simple stretching of the wing, (either
due to experimental procedures or an intraspecific population or developmental
process) an affine transformation approach was also applied. The
calculation of the affine transformation of landmarks was carried out according
to Rohlf and Slice, (1991). The affine transform
and subsequent calculations of averages and differences between group averages
were carried out within an MS-DOS APL workspace, AFFINE, implemented by
the first author in STSC APL 8.0.
Distance based methods were used to calculate phenetic trees of
similarity between wing shapes. The C code for the distance based
tree building algorithms, NEIGHBOR, FITCH and KITCH of the PHYLIP program
package, was obtained from the PHYLIP FTP site and compiled on a SPARCstation®
10 (Felsenstein, 1989). In all cases the topography
of a UPGMA tree, calculated using the NEIGHBOR algorithm, concurred with
the contemporary branch option of the KITCH algorithm and only the more
interpretive KITCH results are reported.
The program Planar 1.0 which was used for least square alignment, averaging of wing landmarks and discriminant function analysis is available as a zipped self extracting MS-Dos utility, plansfx.zip, at a WWW downloading site (URL http://www.bio.umass.edu/biology/kunkel/). The APL workspaces used in this paper, afine_apl.zip, are also available at the above site for anyone with access to the calculating environments: STSC APL or APL+ or APLSE.
Additional information about the tra-2 gene is available from a Flybase (1996) Gene Report on locus TRA-2 or via a query of Genebank for tra-2: (Genebank locus TRA-2 protein) or tra-2 Protein D: (Genebank locus TRA-2 protein D) .
Sex dimorphism of wings in Drosophila.
There is significant sexual dimorphism in wing vein
landmark locations in wild-type strains of D. melanogaster.
Fig. 2 illustrates a typical result of sampling
wings from a culture of the Canton S wildtype strain. In this figure the
locations of 13 landmarks of males and females are depicted as centroids
plus 95% confidence ellipses of landmarks aligned by the least square algorithm
of Siegel (1981). The dimorphism is most evident
in examining the male (circles) and female (filled squares) averages and
CI ellipses for landmarks five and thirteen. For these landmarks the confidence
intervals for the raw landmarks are markedly askew. A 95% confidence
ellipse for aligned landmark five, the intersection of vein II with the
wing margin, is depicted for the sexes combined (Fig.
3) and for males and females separately (Fig.
4).
Consistency of dimorphism among wild type strains.
Consistency of overall wing sexual dimorphism
A Procrustes distance matrix (and its approximation
in tangent space) (Table I) between male
and female D. melanogaster of three wild type strains was calculated
using the tpsSmall program of Rohlf (1997C).
A tree of difference-between-strains-and-sexes, Fig.
5, was constructed by applying the Fitch Margoliash algorithm (Felsenstein,
1989), assuming contemporary tips and no negative branches, to the
Table I Procrustes distances. Note that the differences between the
sexes within each strain, in both the table and the resultant tree, are
similar and smaller than the interstrain differences.
In addition, the close to equivalent Procrustes distances and tangent
space approximations allow for the forthcoming more detailed analysis of
dimorphism using the thin plate spline methodology which operates in tangent
space.
Consistency of sex dimorphism of individual landmarks
Three wild type strains, Canton S, University of Tennessee and Porch
88, were examined for sex differences in their wing vein landmarks.
While there were significant random landmark shape differences between
strains, the differences between male and female landmark locations appear
geometrically similar among all three strains (Fig.
6). This apparent difference can now be tested in a more mathematically
rigorous fashion:
First, when the sexes were compared for each wild type strain independently using thin-plate-splines with the program tpsRelw of Rohlf (1997B), these differences were each explainable via the warped coordinate grids as a dilation of the distal portion of the wing blade (Fig. 7A-C). All three wild type D. (S.) melanogaster strains demonstrated a similar sex-dimorphism-related distortion of the wing blade coordinate grid. This sex dimorphism is also conserved across species and subgeneric boundaries, being observed in Drosophila (D.) immigrans, Fig. 7D, but not in Drosophila (D.) virilis, Fig.7E. The slight antero-posterior flexure of the wing between sexes of D. (D.) virilis is seen as a minor component of the total sexual dimorphism in D. melanogaster as will be shown in the principle component analysis of wild type strains below (Fig. 8C).
Second, the uniformity of sex dimorphism across the three wild type D. melanogaster strains considered was established by carrying out a factorial design analysis of 300 wings in which sex, strain and sex-strain interaction could be independently measured, Table IV using tpsRegr (Rohlf, 1997A). The results of this analysis concludes that both main effects, sex and strain, are highly significant separable factors affecting wing shape but there is no significant interaction detectable between them. The main effect, sex, accounted for 25% of the variability explainable by the experimental design. The other main effect, strain, accounted for almost all of the remaining 75% of explainable shape difference.
Third, further analysis of the sex and strain effects on wing shape used tpsRelw to analyze the thin plate spline conversions of the six average phenotypes into five principal components. Three of the five principal components contained 98% of the total explainable variability between the six shapes. The first of these principal components, Fig. 8A, engenders 60% of the explainable variability and corresponds to a shape difference most notable in each of the three individual strains noted above (Fig. 7), the dilation of the distal region of the male relative to the female. The second principal component of sex related wing shape difference engenders 30% of explainable shape difference and corresponds to a shifting of the proximal and distal wing areas away from one another with some rearrangement of the proximal landmarks, Fig. 8B. The third and smallest significant principal component engenders 8% of explainable shape difference and reflects a slight antero-posterior flexure of the proximo-distal wing axis in the male relative to the female, Fig. 8C. These same three principal components of shape also had substantial strain related differences contained within their total variability yet the differences between males and females retained a similar magnitude and polarity for each strain, Fig. 8D. This graphic similarity in magnitude and direction of sex dimorphic warping in the three largest principle warp dimensions of the wings is another reflection of the lack of interaction between strain and sex of Table IV, demonstrated in the similar tree limb lengths between sexes in Fig. 5, the similarity if sexual differences in raw landmark averages of Fig. 6, and the visual similarity of warped grids of Fig. 7A, B, C.
Prediction of sex using wing discriminant functions
The consistent difference between males and females shown above
using averaged wing coordinates begs the question of what significance
this may have for the individual fly. Are the differences in wing
venation, subtle as they are, significant enough to allow identification
of an individual's sex?
Given the ability to create averages of aligned wing landmarks and
confidence ellipses surrounding them, we are set up to give individual
wings a discriminant score based on how close their landmarks come to the
centroids of the confidence ellipses. If we calculate one discriminant
score for the likelihood of being a male and another for the likelihood
of being a female, we can tell the sex of an individual fly using its wing
shape as a determinant. That paradigm was carried out on the three
wild type strains considered above. In this initial case, we simply used
the known sex of the wild type flies to create the basis of male and female
discriminant functions for each wild type strain. We applied these
discriminant functions to the same flies as an initial demonstration of
the resolution of the method, assuming their is little between-culture
within-strain differences in shape ( Fig. 9).
Plots of the log likelihood discriminant scores demonstrate the power of
the tests. The predominant slope in the panels testing for females
is up toward the female pole of the panel, indicating that females received
a higher female-discriminant-score. For males, the slope is toward
the pole of the male discriminant score. The occasional lines which
have reversed slopes indicate the frequency of false positives, when a
wing's sex is incorrectly assigned. Table
II illustrates the success with which these discriminant functions
assign the correct sex to each wing. The discriminant functions for each
strain are able to assign the correct sex to an individual in ~90% of the
cases. A further, more demanding test is to use the discriminant
function on samples independent of the individuals that formed the basis
of the discriminant function. The use of this type of test is described
in the forthcoming section which examines when sexual dimorphism of wings
is established during development.
Genetic regulation of wing sexual phenotype
Since we had demonstrated a sex dimorphism of the wing shape of
several strains of D. melanogaster we sought confirmation of that
observation using the powerful genetic tools available for this species.
We employed the temperature sensitive allele, ts2, of the gene transformer
2 (tra2) to extrinsically control wing sexual phenotype. While
raising tra2ts2 individuals at constant permissive temperature,
18°C, the normal (sex chromosome ratio determined) sexual phenotype
of an individual is expressed. While raising tra2ts2
individuals at constant non-permissive temperature, 29°C, genetic females
are masculinized, judging from the color and shape of the abdomen, the
morphology of the genitalia, and the presence of sex combs on the male
legs. We took such masculinized females and found their wing landmarks
to demonstrate masculinization of the female wing shape (not shown).
Timing of sexually dimorphic development of wings
A temperature shift experiment of tra2ts2 was
used to delineate the temporal limits of required gene activity of tra2
in determining the sex dimorphism of wing shape. Multiple crosses
of Cy tra2+/Cy+tra2ts2 were set up by allowing Curly
winged flies to lay eggs for one day on freshly prepared fly media.
Each day the parent flies were switched to new media creating 8 cohorts
of eggs developing at 29°C. An earlier experiment determined
that transferring to 18°C when wandering 3rd instar larvae first appeared
was too late, since by that time all genetic females had masculinized wings.
By timing the cultures we were able to transfer larvae at measured numbers
of days prior to wandering, covering the mid to late larval stage with
29°C to 18°C transitions.
A culture allowed to develop for the entire life cycle at 18°C
formed a control group, in which genetic females mature as females and
genetic males as males. We used this control group of males and females
to establish our bases of discriminant functions. It also proved
useful to develop discriminant functions for recognizing Curly phenotype
wings, since Curly heterozygotes are not always 100% penetrant and
the limiting Curly phenotype closely resemble wild type (Table
III). Fortunately the discriminant functions in combination with
the superficial wing phenotype allowed identifying a fairly distinct class
of genetically female tra2 homozygotes to examine for the masculinizing
effects of tra2ts2 at 29°C.
Shifting from 29°C (where females are masculinized) to 18°C
(where females can develop as females) during wing imaginal disc development
(Fig. 10), allowed us to identify the phase during
which tra2 activity is a factor. These results indicate that
a functional tra2 gene must be present in mid to late larval stage
to allow correct sexual differentiation of feminine wing shape. However,
from late larva to emergence the activity of tra2 does not have any significant
rescuing effect on the development of wing sexual dimorphism. We
have yet to test whether tra2 is essential during the pupal phase
once it has had its demonstrated action during the larval phase.
This awaits analysis of the more demanding 18°C to 29°C temperature
shift experiment.
The wings of Drosophila melanogaster were shown to be sexually dimorphic in shape. This dimorphism could be observed using several methods for viewing averaged landmark traits. A straight forward averaging of the aligned landmarks, with computation of residual differences between the centroids of males and females, demonstrated the consistency of the result among three wild type strains. A thin-plate-spline of average aligned male and female landmarks produced transformation grids which emphasized the dilation, shifting and flexure of the male wing landmarks relative to those of the female. The present demonstration of sex dimorphism in wing shape is a substantial addition to a previous analysis (Cowley et al., 1986) which used a more traditional quantitative genetic analysis of selected struts between landmarks, many of which were included in the present study. This prior study was notable in testing for, and finding, additive genetic effects, including X-linked variances distinct for each sex. The current study focuses on the benefits accrued from retaining the formal position information that is lost in strut analysis. The current study could potentially benefit from a more rigorous genetic framework and model that quantitative genetic analysis affords. However we focus here on the relative ease with which significant sexual dimorphism was able to be detected using these powerful new mathematical tools and how these techniques can be further used during experimentation on sex dimorphism.
While the distinct principal components of wing shape discovered were highly significant, there is no proof that such separate components need be regulated by separate or single growth factors. As in all principal component analyses, the components are not necessarily rationalizable. It is comforting that the theme of the major principal component (dilation of the distal wing blade of the male relative to the female) seemed to be identical and directly visible when examining the three individual wild type strains. A major conclusion of the factorial design analysis of 300 individual wings from three wild type strains is that there is no significant interaction between the main effects of sex and strain. This is of major significance because it is the sex-strain interaction term that one would examine in a genetic screen for the effects of genes that control the wing-specific sexual dimorphism of wing shape.
The difference between male and female wings was substantial enough to provide a basis for sexual discrimination in experiments requiring determination of sexual phenotype of individual wings. Of the three wild type strains studied, Canton S, U. Tenn. Ag. Station and Porch 88, the oldest established strain, Canton S, showed the smallest incidence of false positive sex prediction. Although a significant phenomenon, the greater predictability of the older strain was an a posteriore observation and needs rigorous confirmation by further observation. One might expect that many genes contribute to the large interstrain differences indicated by Table I. Recent wild type strains, particularly ones sampled closer to their origination date, should retain more heterozygosity of genes which determine wing shape. Canton S has been maintained in many labs without efforts to maintain its genetic robustness, and would be expected to be nearly isogenic. While isogenicity may lead to greater environment associated variability, such sensitivities may have been lost from the Canton S strain.
It was particularly helpful to use one of the sex determination genes, transformer-2 (tra2), to confirm that wing sexual difference was under the same regulation as other sexually dimorphic traits. Using the temperature sensitive allele, tra2ts2, wings that develop in the continuous permissive temperature, 18°C, retain the sexuality defined by their sex chromosomes. At the non-permissive temperature, 29°C, the chromosomal females develop male morphology, including the shape of male wings. Of course if the traditional sites of sexual dimorphism, the genitalia or sex combs, are accessible, they are superior to wings as a way of sexing the organism. However, in our instance we were interested in when the subtle differences between male and female wings develop. By using the ts2 allele of tra2 we were able to show that the sex specific morphology of the wing develops during the mid-to-late larval period, a wing disc growth period. This is in contrast to the control of the development of the genitalia's dimorphism, which occurs in the pupal period (Epper & Bryant, 1983) and yolk protein synthesis in female fat body which requires tra2 function in the adult (Belote et al., 1985). Both these and other works imply that tra2 must be active at the time the regulated process is itself developing. We therefore conclude that the sexual dimorphism of the wings is developing in the imaginal discs during mid to late larval life. The dimorphism was found to stem from a dilation of the distal vs basal part of the female wing. This implies that the subtle difference in wing shape could be based on small differences in regional growth rates of the larval wing imaginal discs.
The growth of the wing imaginal disc during the larval stage has
been shown to be under regulation by Decapentaplegic (Dpp),
a member of the TGF![]() family of growth factors (Burke and Basler, 1996).
Furthermore, the receptor of Dpp, Thick veins (Tkv),
and a cofactor in Dpp action, Schnurri, are also necessary
downstream elements required for growth of local patches of wing.
A proximal-distal patterning of the wing has been ascribed to wingless
(wg) which has a local mitogenic effect in the hinge region but
a repatterning effect in the wing blade with only a secondary effect on
proliferation (Neumann and Cohen, 1996). Thus
there seem to be several growth factors operant in the wing imaginal discs
which could be regulated in a sexually dimorphic manner to achieve the
multiple differences we have observed.
family of growth factors (Burke and Basler, 1996).
Furthermore, the receptor of Dpp, Thick veins (Tkv),
and a cofactor in Dpp action, Schnurri, are also necessary
downstream elements required for growth of local patches of wing.
A proximal-distal patterning of the wing has been ascribed to wingless
(wg) which has a local mitogenic effect in the hinge region but
a repatterning effect in the wing blade with only a secondary effect on
proliferation (Neumann and Cohen, 1996). Thus
there seem to be several growth factors operant in the wing imaginal discs
which could be regulated in a sexually dimorphic manner to achieve the
multiple differences we have observed.
Based on these results, we surmise that other subtle sexual dimorphic phenomena, which may involve minor differences in relative regional dimensions, may be regulated in mid to late larval period, during imaginal disc growth. This may be an important mechanism affecting, in the case of wings, mate choice and recognition of individuals from identical populations. It would also be interesting to reexamine some of the other sex dimorphic traits such as the genital segments, which are transformed superficially during the pupal period to appear male based on pigmentation, to see if their are shape components that are regulated during growth of the genital disc during the larval stage.
The functional consequences of sexually dimorphic wing morphology in Drosophila are not spoken to by our data. Two hypotheses of functional roles for the dimorphic structures warrant further examination. First, the female wing design confers load bearing superiority. Second, structural components of the male wing design are required for wing-based song. Both of these hypotheses would require careful scrutiny, especially since the differences between male and female, however statistically significant, are quite subtle. The female abdomen, full of eggs, would be well served by a wing with greater lifting capacity. This might be accomplished, for instance, by strengthening the proximal wing hinge area, which might bear the majority of strain in a lifting situation. Thus a combination of growth regulated by X/A and regional activity of growth regulators could provide a wing design for a different load bearing situation. This could also derive a wing whose vibrational properties are tuned precisely for male wing-song.
Whether the sexually dimorphic wing structures are in fact functional is still unclear. The conservation of a particular type of wing dimorphism across subgeneric borders that we demonstrated above is an indication that the dimorphism may confer a selective advantage to some species. Their is obvious potential for using this important naturally occurring dimorphism, which can now be followed with our modern landmark oriented approach, for expanding our understanding of subtle genetic differences. Indeed, our discovery of the wing sexual dimorphism in combination with the rapid expansion of our understanding of the molecular genetics of Drosophila wing development will aid in future investigation of the function, evolution and maintenance of this integrated functioning unit.
Brian Bettencourt was supported as a Howard Hughes Undergraduate Research Fellow during his participation in this research. We are indebted to the late Phil Ives who provided us with the initial cultures of Drosophila sp. used in this work. We appreciate discussions with Mary Bownes which inspired the use of the tra2ts2 strategy in our experiments. We are grateful to Randal Phyllis for sharing his Drosophila handling and genetics expertise with us. John Nambu suggested the discussion point relating wing shape to wing song. We are indebted to F. James Rohlf for his efforts in providing software for morphometric analysis and for helpful comments on this manuscript. The writing of Planar 1.0 was supported by a grant from the USDA APHIS.