"For tautog aqua culture to advance, the effect of diet quality on egg production, larval survival and larval growth must be quantified" (Perry and Ramseyer, 1997). This request for analytical assistance requires a special laboratory that is familiar with dealing with both biochemical analyses and the concepts of morphological growth and development of embryos. The maternal effects of the female diet and larval diet on the success of the offspring can be transmitted in several ways. First, the health of the mother may be affected and her general health may determine egg quantity and quality. Second, the maternal effect on egg quality may have its effects on embryonic processes that precede the utilization of stored egg reserves (lipid, yolk protein) or the ability to access those reserves. Finally, the maternal effect may be exhibited in the reduction or modification of the reserves that are stored in the egg. In addition the quality of the larval and juvenile diets may directly affect their development.
We intend showing that a battery of basic biochemical measurements combined with simple landmark measurements and modern morphometric analysis can assist the assignment of a quantitative index of developmental state to embryonic and larval stages of fish. This battery of measurements will use modern inexpensive technology which can be applied to individual embryos. This approach will allow factorial experiments to be designed to evaluate various aspects of aquaculture methods to the rate and uniformity of larval growth. Factorial experiments will be designed to test the effectiveness of defined applied nutritional regimes. The dietary factors to be tested include various live algal species cultured at the Milford NMF Laboratory which will be analyzed with respect to their spectrum of polyunsaturated fatty acid and amino acid contents. The timing of conversion to a less expensive commercial fish diet will be a factor in the experimental design. The contrasting diets will be built from analyzed components with the C3 and C6 polyunsaturated fatty acids as major variables.
My laboratory is prepared to carry out all the methodology required to satisfy the Perry and Ramseyer request for technical assistance in studying dietary fatty acid and amino acid composition on the growth rate and body composition of larval tautog and reproductive success of the adults.
Biochemical Methods.
The Kunkel lab is familiar with the techniques of lipid extraction,
amino acid analysis and gravimetric determination of % lipid in biological
samples (Kunkel and Pan, 1976). We
will prepare lipid extracts of the proposed dietary constituent algae provided
by the Milford Lab and add appropriate antioxidants to the lipid extracts.
The polyunsaturated profiles will be analyzed under the direction of Associate
Professor Eric Decker of the U. Mass. Department of Food Sciences (endorsement
letter attached). The protein fraction will be hydrolyzed in 6N HCl and
the amino acid content analyzed by the analytical facility of the Molecular
and Cellular Biology Program at $50 per sample.
Analysis of the protein, RNA and DNA content of the resultant eggs and larvae will be carried out by established protocols in the Kunkel laboratory. Analysis of calmodulin (CaM) content per embryo will be carried out using dot blot immunochemical test with available anti-CaM serum which has broad species specificity (Zhang & Kunkel, 1991). Analysis of lipovitellin per embryo will also be carried out by dot blot analysis based on purification of Tautog lipovitellin (Lv) and raising a specific anti-Lv serum in a rabbit (Hartling, Pereira and Kunkel, 1997).
Growth Measurements.
Morphometrics has experienced a revolution in the past decade.
The old statistical techniques are being abandoned in favor of a new approach
that allows one to deal with the size and overall shape of an organism.
New tools for examining shape have been developed and are freely shared
by an active group over the internet: (URL: http://life.bio.sunysb.edu/morph/).
The new techniques focus on landmarks and measure the distortions that
occur in a set of homologous landmarks as an organism develops or evolves.
This approach can be applied to either 2-D profiles or actual 3-D coordinates
of specimen landmarks. I will develop this proposal for the 2-D profiles
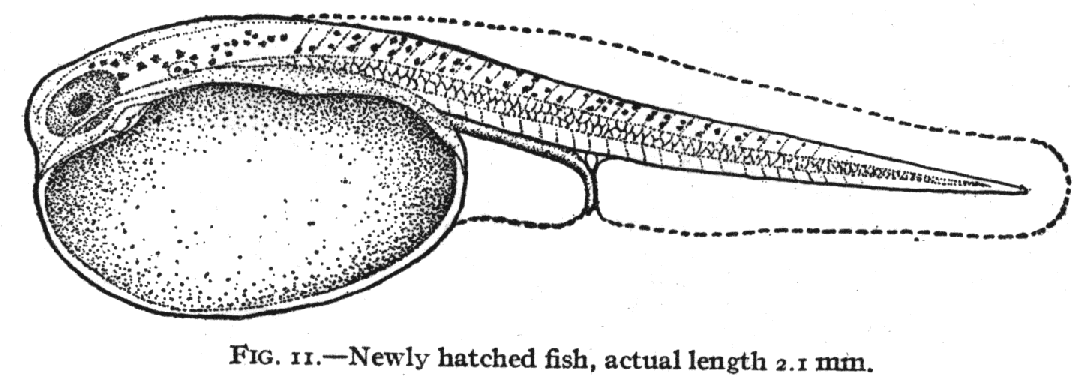
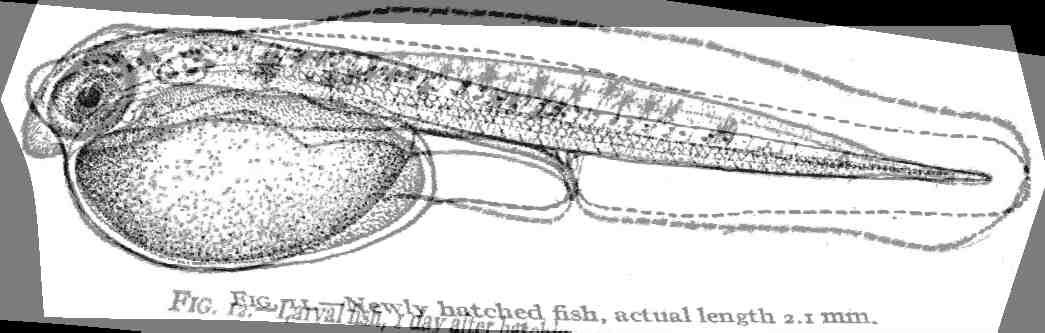
of fish that students of fish development are familiar with using ( Kuntz
and Radcliffe, 1917, fig. 11).

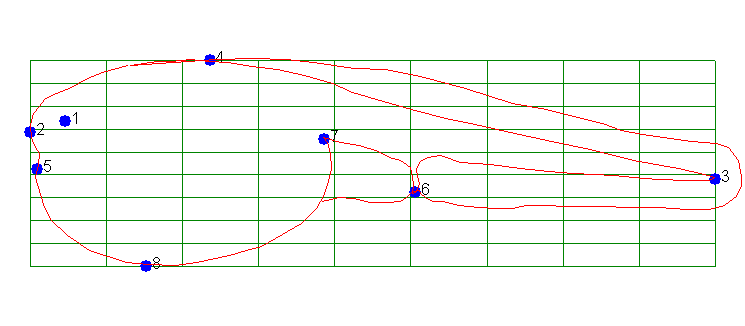
We can do that by defining common landmarks and measuring the degree
of distortion that is needed to transform one image into the other.
To use Fig. 11 as a reference we define the landmarks and establish a grid
pattern that those landmarks reside in:
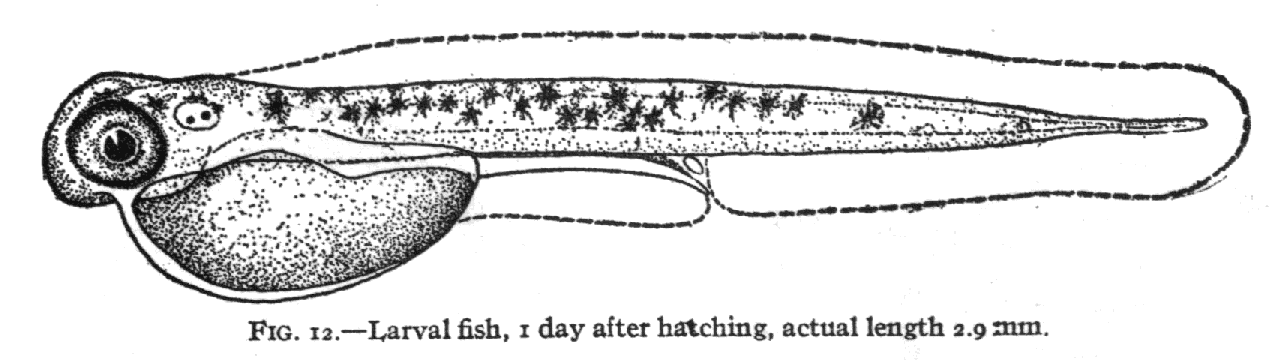
By using the mathematics of thin-plate-splining which is made available
to us through the software of James Rohlf of SUNY Stoneybrook, we can compute
the distortion of the grid that needs be applied to change the shape of
Fig. 11 into the shape of Fig. 12.:
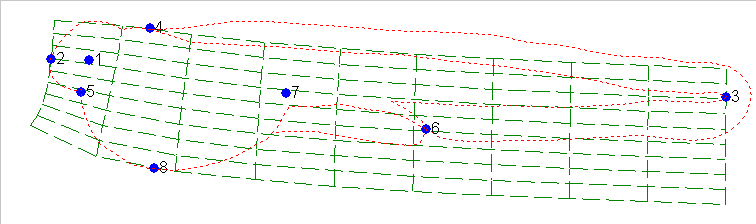
As one can see the grid pattern of Fig 11 needed to be squeezed and
twisted in several ways to achieve the shape of Fig 12. This can
also be illustrated using the vectors which transform the two figures:
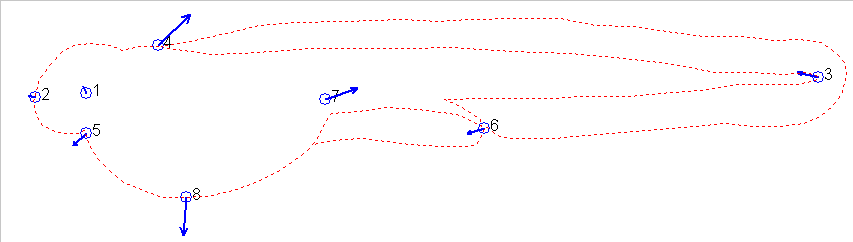
These vectors are another measure of the difference between one stage
and another and can be used to measure how different (advanced or retarded)
one stage is relative to another or relative to a series of stages used
as references. In effect, one can measure the shape difference between
any two individuals. The shape difference is independent of size
difference, which can also be captured as the amount of isometric scaling
factor that needs to be adjusted to allow the landmarks to be superimposed
most closely.
For the above two figures, or any set of n figures, we can also calculate an average image based on the set of 8 landmarks:
The image average as illustrated above is potentially a useful tool
that might be applied to individuals which according to age or sorting
are all considered to be nominally the same stage. A good diet
might be defined as one that produces the smallest variation about the
mean in a synchronous cohort of developing larvae. The stage image
average in this case would also give one a basis for discriminating individuals
close to that stage. By using the dispersion (covariance) matrix
of the averaged landmarks one can also establish a discriminant function
which gives an unknown larva a score indicating how closely it conforms
to that stage. With a series of stages, L1-Ln
, defined at half day or 8 hour intervals one could quantitatively score
the development of any individual larva as to its proximity to stages
L1-Ln of development.
The series of scores, L1-Ln, for each individual,
plus scores for dry weight, total protein, total RNA and DNA, and two key
yolk proteins, Lv and CaM, could be very effective tools for comparing
the effects of diets on larval development. CaM has been shown in
other organisms to occur in large amounts in eggs and to remain high during
the rapid cell division phase of embryonic development, prior to Lv utilization.
A Lv measure would allow comparing the effects of maternal and larval diets
on the yolk utilization phase which stretches from hatching through the
establishment of normal feeding. Following CaM in combination with
Lv might be a useful tool in determining any effects of maternal diet on
yolk composition. These biochemical measures of development are more
elaborate and expensive to carry out and must be evaluated as to whether
they improve over the simpler morphometric measure of development.
The Kunkel Lab is experienced in measuring Lv, CaM and RNA on individual
embryos (Hartling et al., 1997;
Zhang and Kunkel, 1991; Iyengar
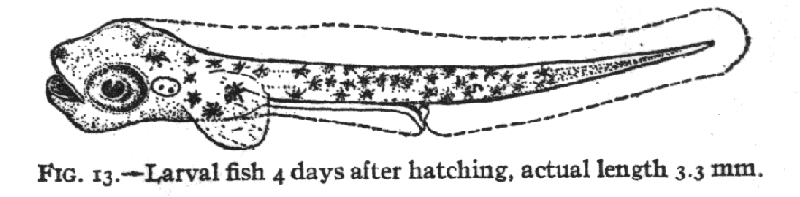
and Kunkel, 1995). As the larva develops and particular landmarks
appear and disappear, a given stage may be defined by a new set of measures
and landmarks (i.e. Kuntz and Radcliffe, 1917,
fig. 13) with old irrelevant measures and landmarks dropped to improve
the discrimination for the current span of development.
The above measures can be used in a traditional factorial experimental
design which can be analyzed with available statistical packages.
Thus an observation vector on individual larva 'k' from maternal diet 'i'
fed larval diet 'j' might include:
Yijk = Hatch-Age, DriWeight, Protein, Vg, CaM, RNA, DNA,
Size, Score-1, Score-2, ..., Score-n.
This linear equation can be expressed in matrix form:
Y(ijk) = Xß
,
where Y(ijk) is the kxp observation matrix of p measures
on k individual larvae, X is the design matrix specifying the treatments
and interactions assigned to each treated larva and ß
is the parameter matrix to be estimated by the experiment and tested for
significance. A simple 6x6 unit design matrix with two maternal diets
and three larval treatments is presented in the following table:
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
In addition one could work with the Analysis of Dispersion Model (Rao,
1965) to ask such questions:
(1) Given Age, DNA and Size is any additional information provided
by Score-1 and 2?
(2) Given Larval Size is any additional information provided
by DNA, RNA, Protein, Vg or CaM?
Such questions are important in establishing a minimal protocol for
comparing the effectiveness of applied diets. There is no use in
applying multiple and costly assays if they do not supply additional information
above and beyond simpler measures such as Larval Size or one or more Shape
scores.
The Kunkel laboratory has a published history and capability of applying
both biochemical and morphometric approaches to the study of development.
entrez
author search on kunkel-jg . We are particularly interested
in applying our experience with measuring biochemical and morphometric
properties of organisms during development. We intend to use this
opportunity to demonstrate the usefulness of using a combination of biochemical
and morphometric assays to establish the effect of dietary factors
The above protocols and experiments depend on a supply of eggs, timed
embryonic and larval samples at appropriate times from Dean Perry and Laurel
Ramseyer of the Milford NMF Laboratory. Initially, during the first year,
we will need unfertilized eggs for purification of tautog Lv and making
our anti-tautog-Lv-serum and testing our anti-CaM-serum. Milford Lab will
also need to provide samples of fixed whole embryos and larvae at characteristic
stages or ages (10 embryos per age/stage) so that we can establish the
scoreable landmarks for each stage. In year two we will be applying our
staging and analytical tools to the analysis of the factorial design experiments
on dietary effects. The Milford lab will be integral to the experimental
design decisions based on the analyses of the diet constituents and their
best judgment about appropriate diet treatment contrasts for the developing
embryos. They will establish the timing of sampling and provide live, frozen
or fixed samples of embryos and/or larvae, depending on the assays we are
carrying out.
The Milford NMF Lab has provided two lists of samples, one for polyunsaturated
fatty acids (PUFA) and another for amino acids (AA), that it would like
to be analyzed. The following two tables characterize the samples which
will be provided by the Milford Lab for analysis by the UMass Lab:
| PUFA Analysis Prey items and larval tautogs | Samples | Type |
|---|---|---|
| Mussels | 3 | macro |
| Crabs | 3 | macro |
| Commercial Diets | 3 | macro |
| Unicellular algae | 3 | macro |
| Rotifers | 4 | macro |
| Artemia | 5 | macro |
| Fertilized Eggs
(1-4 gms) |
2 | macro |
| Larvae -
pool of 10 fish x 6 tanks x 5 weeks |
30 | micro |
| Larvae - after 5 weeks,
sample every 2 wks 4-6 x 6 |
24-36 | micro |
| Larvae - 1 day before start feeding
pool of 10 fish x 6 tanks |
6 | micro |
| Larvae - 2 days after yolk-sac absorption
pool of 10 fish x 6 tanks |
6 | micro |
| Total | 91-103 | all |
| AA Analysis of larval tautog | Samples |
|---|---|
| Fertilized eggs | 2 |
| Newly hatched
pool of 10 larvae x 6 tanks |
6 |
| Before metamorphosis | 4 |
| After metamorphosis | 4 |
| Total | 16 |
YR-1 YR-2 2-YR 98-99 99-00 98-00 1 JG Kunkel $0 $0 $0 Personnel: 1 graduate student ($13.08/hr) $13,603 $13,604 $27,207 Geo Fringe benefits ($1.525/hr) 1,586 1,586 3,172 1 undergraduate student trainee 500 500 1,000 Services: PUFA analysis 300 1,000 1,300 Amino Acid Analysis 100 800 900 Antibody Production 1,000 0 1,000 Equip: Trinocular dissecting scope. 4,000 0 4,000 CCD Camera 0 500 500 Dedicated computer 0 2,000 2,000 Frame grabber Board 0 1,000 1,000 Supplies: Chemicals, immunologicals, glassware 3,000 3,000 6,000 Total: $24,089 $23,980 $48,069
| Year 1 | 1st 6 months | a. Graduate student training phase in PUFA analysis.
b. Purification of tautog Lv. c. Characterization of tautog CaM reactivity in dot blot assay. d. Analysis of diet PUFA components. |
| 2nd 6 months | a. Antiserum production.
b. Development of morphometric analysis. c. Preliminary PUFA analyses on various aged fish. |
|
| last 3 months | a. Characterization of anti-tautog-Lv and developing dot blot assay. | |
| Year 2 | 1st six months | a. Collect and assay tautog growth samples for all feasible assays. |
| 2nd six months | a. Prepare results for publication. |
| Born: Oceanside, New York, August 17, 1942. | ZZ# XXX-XX-YYYY |
| Home: 74 Stony Hill Rd.
Amherst MA 01002 |
University: Biology Department
University of Massachusetts Amherst MA 01003 |
| Home Phone: (413) 253-3391 | Office/Lab Phone: (413) 545-0468
Email: joe@bio.umass.edu URL: http://www.bio.umass.edu/biology/kunkel/ |
Columbia College, New York, New York, Zoology A.B. 1964
Case-Western Reserve University, Cleveland, Ohio, Biology Ph.D. 1968
Dissertation: Control of Cockroach Development
Awards and Honors:
Marine Biological Laboratories Corporation Member, elected 1996; NSF Graduate Fellow, Biology, Case Western Reserve University, 1967-68; R.C.A. Scholar in Chemistry, Columbia College, 1961-62; Columbia University Scholar, Columbia College, 1960-64; New York State Regents Scholar, Columbia College, 1960-64; Bnai Brith Scholar, Columbia College, 1960.
Positions and Professional Experience:
| Sabbatical, National Vibrating Probe Facility, MBL, Woods Hole, MA | 1993-94 |
| Member, Organismal and Evolutionary Biology Program | 1996-present |
| Full Professor, University of Massachusetts at Amherst | 1985-present |
| Adjunct Professor of Entomology, University of Massachusetts. | 1985-present |
| Sabbatical with B. Lanzrein, Zoological Institute, U. of Berne, Switzerland, | 1985-86 |
| Member, Cell and Molecular Biology Program | 1983-present |
| Associate Professor of Zoology, University of Massachusetts. | 1976-85 |
| Sabbatical with Alan C. Wilson, University of California at Berkeley | 1977-78 |
| Assistant Professor of Zoology, University of Massachusetts, Amherst | 1970-76 |
| Lecturer in Biology, Yale University | 1969-70 |
| NIH Postdoctoral with Gerry R Wyatt, Yale University | 1968-70 |
| Postdoctoral in Biometry, Case Western Reserve University Medical School | 1968 |
| Graduate Research Assistant, Biology Department, CWRU, Cleveland | 1964-68 |
| Research Assistant to Arthur W. Pollister, Columbia University, NY | 1963-64 |
| Research Assistant to Francis J. Ryan, Columbia University, NY | 1963 |
Rolf Koenig, Postdoctoral Research Associate, 1985-86.
Elizabeth S. Bowdan, Senior Research Associate, 1986-1993.
Joseph Zydlewski, Predoctoral Research Associate, 1998-present.
PhD Students: Raymond Duhamel PhD '79, Don Wojchowski PhD '84,
Sharon Karpells PhD '88, Yujun Zhang PhD '92, Rachel Dompenciel PhD '93,
Anand Iyengar '96, Ruth Hartling, Ellen Faszewski
Kunkel JG and JH Nordin. 1985. Yolk Proteins. in Comprehensive Insect Physiology, Biochemistry and Pharmacology. Chapter 4, Vol.I, eds. GA Kerkut and LI Gilbert, Pergamon Press, pp 83-111.
Kunkel JG. 1986. Dorsoventral currents are associated with vitellogenesis in cockroach ovarioles. in Ionic Currents In Development ed. R Nuccitelli, Alan R Liss Publ., pp 165-172.
Kunkel JG, R Koenig, H Kindle and B Lanzrein. 1986. The role of ions in vitellogenesis and patterning in insect oocytes. Advances in Invertebrate Reproduction 4: 101-108.
Kunkel JG (1988) Analytical Immunological Techniques. Chapter I in Immunological Techniques: Arthropods. Edited by LI Gilbert. Springer Verlag, pp 1-41.
Kunkel JG. 1991. Models of pattern formation in insect oocytes. In Vivo
5: 443-456.
Duhamel RC and JG Kunkel. 1983. Cockroach larval-specific protein (LSP), a tyrosine-rich serum protein. J. Biol.Chem. 258: 14461-14465.
Wojchowski DM, PA Parsons, JH Nordin and JG Kunkel. 1986. Processing of Provitellogenin in Insect Fat Body: a role for High-Mannose Oligosaccharide. Dev. Biol. 116: 422-430.
Koenig R, JH Nordin, CH Gochoco and JG Kunkel. 1988 Studies on ligand recognition by vitellogenin receptors in follicle membrane preparations of the German cockroach Blattella germanica. Insect Biochem. 18, 395-404.
Kunkel JG and E Bowdan. 1989. Modeling currents about vitellogenic oocytes of the cockroach, Blattella germanica. Biol. Bull.176(S): 96-102.
Anderson M & JG Kunkel. 1990. Cleaning insect oocytes by dissection and enzyme treatment. Tissue & Cell 22: 349-358.
Bowdan E & JG Kunkel. 1990. Patterns of ionic currents around the developing oocyte of the German cockroach, Blattella germanica. Developmental Biology 137: 266-275.
Kindle H, B Lanzrein and JG Kunkel. 1990. The effect of ions, ion channel blockers, and ionophores on uptake of vitellogenin into cockroach follicles. Developmental Biology 142: 386-391.
Siegel E, R Baur, JG Kunkel, H Kindle, and B Lanzrein. 1990. Demonstration of a voltage dependent calcium current in follicles of the cockroach, Nauphoeta cinerea. Invert. Reprod. Devel. 18: 159-164.
Zhang Y and JG Kunkel. 1992. High abundance calmodulin from Blattella germanica eggs binds to vitellin subunits but disappears during vitellin utilization. Insect Biochem. Molec. Biol. 22: 293-304.
Zhang Y and JG Kunkel. 1992. Program of F-actin in the follicular epithelium during oogenesis of the German cockroach, Blattella germanica. Tissue & Cell 24: 905-917.
Zhang Y and JG Kunkel. 1994. Most egg calmodulin is a follicle cell contribution to the cytoplasm of the Blattella germanica oocyte. Developmental Biology 161:513-521.
Anderson M, E Bowdan and JG Kunkel. 1994. Comparison of defolliculated oocytes and intact follicles of the cockroach using the vibrating probe to record steady currents. Developmental Biology 162:111-122.
Bowdan E and JG Kunkel. 1994. Ionic components of dorsal and ventral currents in vitellogenic follicles of the cockroach, Blattella germanica. J. Insect Physiol. 40:323-331.
Kunkel JG and PJS Smith. 1994. Three-dimensional calibration of the non-invasive ion probe (NVPi) of steady ionic currents. Biol. Bull.
Kunkel JG and E Faszewski. 1995. Pattern of potassium ion and proton currents in the ovariole of the cockroach, Periplaneta americana, indicates future embryonic polarity. Biol. Bull. 189:197-198.
Iyengar AR, and JG Kunkel. 1995. Follicle cell calmodulin in Blattella germanica: Transcript accumulation during vitellogenesis is regulated by juvenile hormone. Developmental Biology 170: 314-320.
Kunkel JG and EE Faszewski. 1995. Pattern of potassium ion and proton currents in the ovariole of the cockroach, Periplaneta americana, indicates future embryonic polarity. Biol. Bull. 189:197-198.
Hartling, RC, JJ Pereira, and JG Kunkel. 1997. Characterization of a heat-stable fraction of lipovitellin and development of an immunoassay for vitellogenin and yolk protein in winter flounder (Pleuronectes americanus). J. Exp. Zool. 278: 156-166.
Hartling RC, JJ Pereira
and JG Kunkel. 1997. Characterization of a heat-stable fraction of lipovitellin
and development of an immunoassay for vitellogenin and yolk protein in
winter flounder (Pleuronectes americanus). J. Exp. Zool. 278:
156-166.
Iyengar, A. R., and J. G. Kunkel.
1995. Follicle cell calmodulin in Blattella germanica: Transcript
accumulation during vitellogenesis is regulated by juvenile hormone. Dev.
Biol. 170:314-320.
Kunkel JG, and ML Pan. 1976.
Selectivity of yolk protein uptake: Comparison of vitellogenins of two
insects. J. Insect Physiol. 22: 809-818.
Kuntz, A. and R. Radcliffe. 1917. Notes on the embryology and larval development of twelve teleostean fishes. Bull. U. S. Bur. Fish. 35:87-134.
Perry, D. and L. Ramseyer. 1997. Effect of Dietary Fatty Acid and Amino Acid Composition on the Growth Rate and Body Composition of Larval Tautog (Tautoga Onitis) and on the Reproductive Success of Adult Tautog. Project Proposals to NOAA/CMER.
Rao, C. R. 1965. Linear Statistical Inference and Its Applications. John Wiley, New York, 522 pp.
Zhang, Y., and J. G. Kunkel. 1991. High abundance calmodulin from Blattella germanica eggs binds to vitellin subunits but disappears during vitellin utilization. Insect Biochem. 22:293-304.